Authors: Ziyi Xu, Chun Chen
Editor: Ginger Ding
In the 11 years from its debut in 2009 to its IPO on the Hong Kong Stock Exchange in 2020, Kintor Pharmaceutical has had 5 clinical-stage products under development and carried out multiple phase I-III clinical trials across three continents covering a broad spectrum of diseases. The drug candidates include second-generation androgen receptor (AR) antagonist Proxalutamide, AR antagonist Pyrilutamide, fully human monoclonal antibody GT90001 (ALK-1), and others. The milestones that Kintor has achieved are due to team members’ perseverance, insight into market demand and the embracing of team diversity.
Today’s interviewees are from the Kintor executive team. One is an investment bank elite and the other is a doctor who returned to China after working in the US.
Deputy General Manager Dr. Jie Chen and CFO Lucy Lu
MyBioGate: Could you briefly introduce yourselves and talk about how you started at Kintor?
Lucy Lu: Since graduating from Peking University, I have been working in the investment banking sector. Based in Hong Kong, I spent 11 years with UBS Investment Banking Division and I was an Executive Director in the Asian healthcare team when I left in early 2018. At that time, I received an invitation from GF Securities (one of the top 5 Chinese brokers) to contribute to the development of local securities firms. I joined GF as a general manager and Managing Director in charge of overseas investment banking business and covering China’s healthcare industry. In addition, since 2014, I have been a signing Principal for Hong Kong IPOs. Although I have been engaged in finance-related work, I have a strong interest in science. When working in investment banks, I deliberately chose the healthcare sector and helped many healthcare companies go public.

On May 22, 2020, Kintor (9939.HK) was successfully listed on the Hong Kong Stock Exchange
As for Kintor, I’ve known the founder, Chairman and CEO, Dr. Tong Youzhi, since my years in UBS. In 2019, when Kintor was looking for a CFO, Dr. Tong sent me an invitation. In the process of getting to know the Kintor team, I felt that the company was very down-to-earth and the core management team was full of enthusiasm. Kintor believes it is their responsibility to develop novel therapies for patients. I have a faith in this belief and this was the most important reason that I joined Kintor.
Dr. Chen: I graduated with a Ph.D. in organic chemistry from the Shanghai Chinese Academy of Sciences. After graduation, I went to Texas Southwest Medical Center for post-doctoral studies. My main research and development work were on the total synthesis of natural products and the design of medicinal chemical molecules.
When I returned to China in 2015, I thought that working in a scientific research institute might be a good choice for me. Later I realized that the work at academic settings was mostly basic research, and there was a large gap from translating the basic research into industrial science. I hoped that the training practice of drug design I received in the US could be used in the industry to invent drugs. It coincided with the fact that in 2016 that Kintor was looking for an expert to be in charge of preclinical drug design. As this position matched my background, I joined Kintor and was responsible for the molecular design and discovery of preclinical drugs. Since November 2018, I have taken over the position of Deputy General Manager, responsible for the company’s daily operations and management.
MyBioGate: Both of you faced a transition after starting at Kintor, from finance to drug development or from R&D to management. Were there any challenges encountered in this transition, and how did you overcome them? What short-term goals you have for career development?
Lucy Lu: My previous job in investment banking was more about diving into a company’s investment highlights and design an attractive investment story to lift a company’s valuation. Since my joining Kintor, I needed to carefully assess what the company needed to do to achieve better results at what timing. At the same time, I need to have a good understanding of our products, the molecule, mechanism of action and pharmacokinetics etc.. Fortunately, both Dr. Chen and my research colleagues have been giving me a lot of coaching so the transition was fantastic. I do enjoy the process of facing challenges and work them out.
Frankly speaking, it is not challenging being the CFO of a startup company. I have absolute confidence in this role. However, I aim to be a scientific CFO. I think a qualified scientific CFO must be clear about the ideas behind the company’s clinical and R&D pipelines, as well as how to face competition upon commercialization. Most of the time I assist my Chairman & CEO to convey our business plan to the market, therefore, I must be a good communicator.
Dr. Chen: For me, the biggest challenge in the transition process came from my background as a scientist. From the perspective of a scientist, what we study and research follows relatively predictable patterns. However, management is about people. People are far more difficult to manage than science as interactions with them are part of a dynamic process. Everyone has different ways of thinking as well as educational backgrounds. As a leader, it is my responsibility to bring everyone together to work toward one goal.
I hope that within 5 years, I will be an outstanding senior executive who is proficient with the entire R&D process and understand the key points and difficulties at each stage. This year, I signed up for a business school course, hoping to improve my R&D management ability through deep learning, apart from my busy working schedule. I push myself to move fast toward my goal.
MyBioGate: Do you have any advice for women who want to enter the pharmaceutical industry in the future?
Lucy Lu: I suggest that women forget about labels of gender in the workplace. Without this distraction, we will be less emotional and handle problems with a rational mind. In addition, in the working environment you should learn to listen, observe and comprehend as much as possible, in this way you will minimize detours along the work. Finally, try to find a mentor who motivates you to achieve the next milestone of your career.
Dr. Chen: In my opinion, the first job you take of course is important, but there are still many options in the future that are not necessarily related to the first job. Thus, you can adjust the direction of future work through continuous observation and learning. I suggest that women should try best to know experienced people in their work and build up relationships. These role models and their experience will help us grow faster.
MyBioGate: Can you tell me about the discovery of Proxalutamide, as one of the company’s lead candidate?
Lucy Lu: In 2007, Dr. Tong’s father was diagnosed with prostate cancer when he was the vice president of Angion Biomedica in the United States. He commuted between New York and Shanghai and began to pay more attention to prostate cancer patients. At that time, there were not many prostate cancer drugs approved in China, and his father and most of the patients were still using the first-generation AR (androgen receptor) antagonist bicalutamide with significant hepatotoxicity. The thought of returning to China to start a business gradually grew in Dr. Tong’s heart. He hoped to find a new generation of safer and more effective drugs for Chinese prostate cancer patients.
Dr. Chen: In 2009, Kintor was established in Suzhou. In the beginning, we conducted a full range of investigations on prostate cancer drug candidates that were approved or in development. We found that Enzalutamide, which was in phase II clinical trial, had preliminary efficacy, but also had some side effects caused by flaws in drug design. With years of experience in drug development, Dr. Tong began to lead the team to design a drug based on the AR structure. In the process of continuous research and development, we found that, unlike the previous mechanism of action, Proxalutamide not only has an antagonistic effect on AR, but also reduces the AR protein expression level. This unexpected discovery led Proxalutamide, as Kintor’s first project, to receive grants from the National Science and Technology Major Project of the Twelfth Five-Year Plan and subsequently received angel financing. In 2013, Proxalutamide filed IND application for prostate cancer indications, and commenced clinical trials in China and the United States in 2015.
MyBioGate: We also have seen that Proxalutamide is being used as the treatment for COVID-19. Would you like to introduce more about this?
Lucy Lu : Proxalutamide was initially developed for prostate cancer and breast cancer treatments. Given the COVID-19 pandemic this year, we have actively explored the use of Proxalutamide for the treatment of COVID-19 . Previously, we reviewed epidemiological studies showing that coronavirus had a faster disease progression rate and higher fatality rate in male patients than in female patients, which also led to speculation whether androgen was related to the coronavirus (SARS-CoV-2) infection. In February, we have collaborated with research institutions to explore the mechanism of action that leads to gender differences among COVID-19 patients. We were delighted to find that AR signal pathway played a role in slowing the disease progression via pre-clinical studies. AR inhibitors are potential novel therapies for the treatment of COVID-19 patients. In July, we have collaborated with a U.S. based company called Applied Biology to carry out a clinical trial of Proxalutamide in men with COVID-19 in Brazil. Recently, we have released preliminary data in Brazil trial, which showed that Proxalutamide had significantly reduced the hospitalization rate and ventilation rate of the patients .
Dr. Chen: We have found that Proxalutamide would down-regulate the expression of ACE2 and TMPRSS2, the two key proteins for coronavirus to enter host cells in lung. The clinical trial in Brazil showed that Proxalutamide had significantly reduced the viral load along the 30-day observation period. More than half of the infected patients who received Proxalutamide treatment were tested negative starting from the 7th day and about 90% of the infected patients were tested negative upon the 30th day. Although it is preliminary data, the result of Proxalutamide in the treatment of COVID-19 is promising. We are actively initiating MRCT (“multi-regional clinical trials”) phase III in countries such as the US and China and hope to obtain an emergency use authorization (EUA) , in this way more COVID-19 patients would benefit from Proxalutamide.
MyBioGate: Apart from Proxalutamide, Kintor also has the drug Pyrilutamide for the treatment of androgenetic alopecia (AGA) and acne. What are the unmet needs in the androgenic alopecia market?
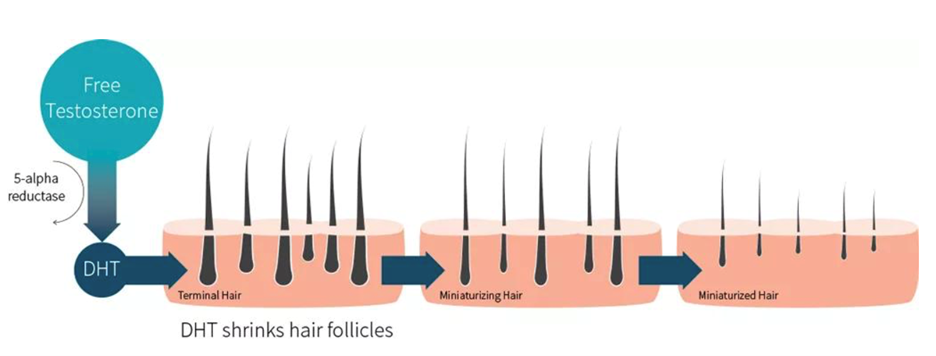
DHT causes hair loss. Source: https://examine.com/
Lucy Lu: We believe that AGA requires long-term continuous treatment, but currently there is few drug with both efficacy and safety in the market. There are more than 200 million people in China who are troubled by androgenetic alopecia, aged between the 20s and 50s, though the average age is trending downwards. Many people may think that androgenetic alopecia only occurs in men. In fact, it also occurs in women. Therefore, our target consumers will be both men and women cross multiple age groups.
MyBioGate: How could Pyrilutamide change the existing competitive landscape?
Dr. Chen: The cause of AGA is very clear, that is, DHT (dihydrotestosterone, one type of androgen) binds to the AR target in hair follicle cells to cause hair follicle cell necrosis, and thus hair loss. At the beginning of drug screening, we hoped to design drugs directly targeting for AR. Our goal was to overcome poor safety and low response rates of existing drugs while ensuring efficacy. After comprehensive inspection, we decided to develop a drug that can act on the surface of the skin, i.e. for local use. Pyrilutamide is an AR antagonist, with excellent efficacy and pharmacokinetics. It can not only prevent the combination of DHT and AR targets to slow down hair follicle necrosis, but also directly act on hair follicle cells through topical use on the scalp. Furthermore, it could be degraded quickly when entering the blood through the skin. According to our data, after 48 hours, the total skin permeability of Pyrilutamide is 1.56%, of which the dermis is 1.05% and the epidermis is 0.33%. After entering the blood, only at the level of nanograms (10-9 grams) can the drug metabolites be detected, which is a strong evidence for the good safety of Pyrilutamide.
Lucy Lu: Our Pyrilutamide has very clear mechanism of action and significant efficacy, and topical use is more patient-friendly. Because of its excellent pharmacokinetics properties, it could meet the high standards on the safety of topical drugs required by FDA and China NMPA, and it is suitable for long-term use among healthy people.
MyBioGate: In the past five years, Kintor has been actively expanding its R&D pipeline. Partners include Pfizer, Sinopharm, Peking University, etc. Meanwhile, the types of drugs have also covered small molecules and biological drugs. In the next 5-10 years, what are Kintor’s plans in development?
Lucy Lu: Kintor is still positioned as an innovative pharmaceutical company. We will maintain our advantages in the field of small molecule innovation and actively expand the R&D pipeline of biological drugs.
In recent years, we have seen more and more successful cases of combination therapies of biologics and small molecules, especially in the oncology sector, which requires a two-pronged approach. In 2018, we licensed in the fully human monoclonal antibody ALK-1 from Pfizer. As a potential first-in-class drug, the data of Phase II clinical trial in Taiwan has shown encouraging results. We will disclose more data at the ASCO GI in January 2021 and are intensively working on the IND applications with FDA and China CDE. In this August, we licensed in the PD-L1/TGF-β dual target antibody developed by US-based company Gensun, and obtained exclusive R&D and commercialization right in Greater China and priority rights outside of Greater China. We hope to apply for the IND with China CDE next year. In addition, the combination of biologics is also very attractive. We hope to explore the combination of ALK-1 monoclonal antibody with Nivolumab, PD-L1/TGF-β dual target antibody, and PD-L1/CTLA-4 bispecific by Alphamab Oncology. We expect the combo strategy can achieve good results, and cover a variety of solid tumors, especially cancer types that are not very effective in current standard care of immunotherapy.

Kintor Landscape
Within 5 years, we hope that Kintor’s core products including Proxalutamide, Pyrilutamide and ALK-1 could hit the market, and create positive cash flow, providing substantial equity returns to shareholders. Within 10 years, we will further enrich the R&D pipeline while continuing to focus on unmet clinical needs and striving to become a leader in the research, development and commercialization of innovative therapies.



0 Comments