Authors: Suzanne Ferguson
The Covid-19 pandemic highlights the critical need for governments and industry to take immediate action to promote faster and more scalable manufacturing platforms, which will require developing cell lines and expression systems that markedly increase the yield of cell-based and recombinant vaccine and antibody manufacturing processes. One such promising gene expression platform is Dyadic International’s filamentous fungal, Thermothelomyces heterothallic, C1 Gene Expression Platform. There is a growing amount of data that proves that the C1 cells, which were originally engineered to produce industrial enzymes at very high yields and enormous commercial scale are able to outperform the existing cell lines such as E.coli, Chinese Hamster Ovary (CHO) cells, insect cells (i.e., baculovirus) and yeast cells in terms of, among other beneficial properties, C1s hyper-productivity, tech transfer and robust growth at flexible commercial scales and total output and cost of goods.
We sat down to speak with Ping Rawson about Dyadic’s ongoing work on its proprietary C1 Gene Expression Platform. Rawson and Dyadic received a lot of positive attention during China Focus SF 2020 during the JP Morgan week. There, she met with a number of Chinese companies, including Jiangsu Hengrui Medicine and initiated a collaboration conversation. On September 17, the collaboration between Dyadic and Hengrui was successfully launched. According to the terms of the collaboration, Hengrui is funding research to engineer Dyadic’s proprietary C1 gene expression system to develop selected Hengrui biologic drugs.

Ping Rawson received her bachelor in economics from Guangdong University of Foreign Studies in 1997 and her MBA from the State University of New York at Buffalo in 2004. Her work experience included Deloitte, Florida Power and Light, and ADT. Currently, she is the CFO at Dyadic International, Inc. in West Palm Beach, where she has worked since 2016.
Dyadic is a global biotechnology company that focuses on the animal and human health pharmaceutical industry to apply its C1 technology. They believe that the C1 technology platform has the potential to be a safe and efficient expression system to help pharmaceutical and biotech companies speed up the development, lower the cost and improve the performance of manufacturing biologic vaccines and drugs to bring more affordable treatments to a greater number of patients, while helping to ease the burden biologic drugs are having on patients and the global healthcare systems, but most importantly saving lives.
Dyadic has built the knowledge, expertise, molecular tools, and technology needed to create and commercialize one of the world’s premiere gene expression systems, the C1 Technology Platform. During C1’s development, they identified two serendipitous mutations: the first changed the morphology of the organism, resulting in high productivity and better growth conditions; the second created the C1 White StrainTM, which allows for the production of purer enzymes and other proteins at high productivity.
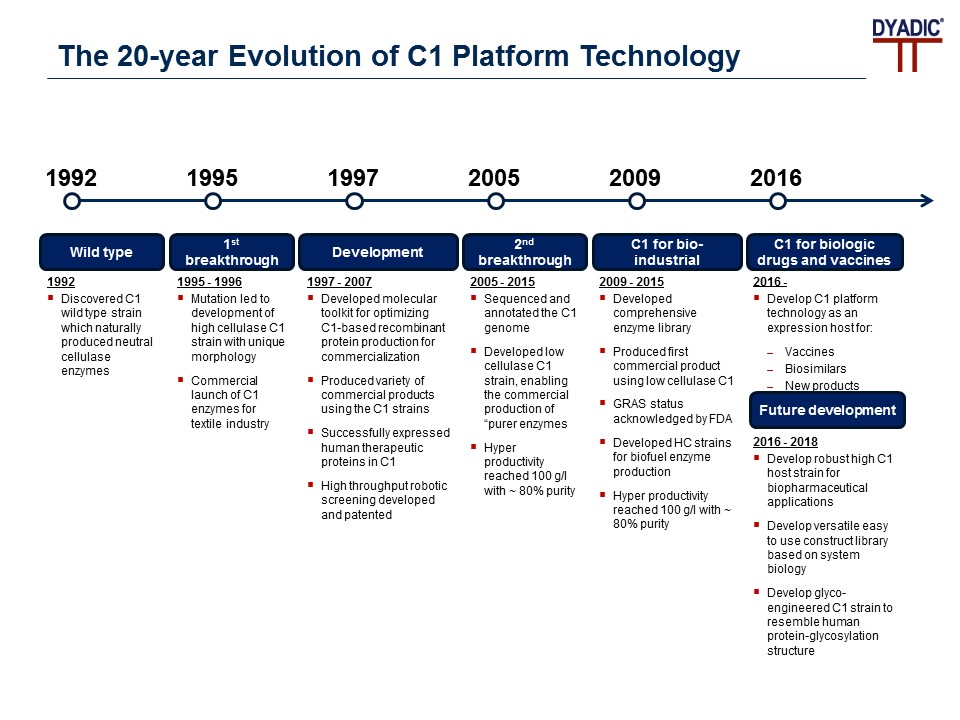
Figure 1: The 20-year Evolution of C1 Platform Technology
Source: https://www.dyadic.com/c1-technology/c1-expression-system/
MyBioGate: How does the C1 expression system bring biologic drugs to the market faster and in larger quantities and reduce the cost of drug developers and manufacturers? What is its technical principle?
The technical principle is the C1 cells grow faster than CHO cells, two hour doubling time vs 24 hours for CHO, and the expression levels that can be reached are higher (2x-5X higher) and the production cycle for C1 is 12-14 days vs 41-54 days for CHO cells. In addition, C1 uses a very low-cost growth media vs CHO cells, and there are no viruses to remove. Therefore, C1 can save two costly additional viral inactivation steps needed with CHO cell produced drugs. C1 is a protein expression and production platform based on an industrially proven and highly engineered thermophilic fungus that has demonstrated low cost, high yield and commercial scalability through numerous collaborations and partnerships. C1 is a proven technology and has been used for more than two decades in industrial enzyme production and demonstrated growth under wide operating conditions for pH and temperature with a low cost of media. C1 is a disruptive platform that shortens the drug development cycle and changes the current approach to producing and manufacturing biologic vaccines and drugs at greater yields and at lower cost using single-use or standard stainless-steel microbial fermenters.
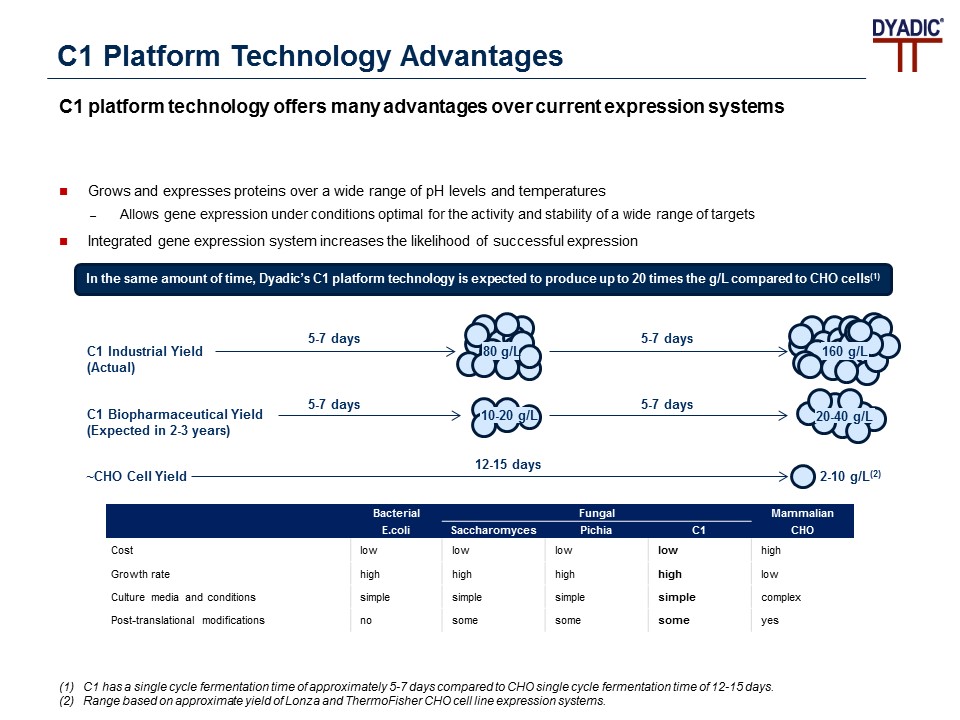
Figure 2: C1 Platform Technology Advantages
Source: https://www.dyadic.com/c1-technology/c1-expression-system/
MyBioGate: We learned that Dyadic is currently working with government organizations in the United States, Europe, and Israel to seek solutions that can produce COVID-19 vaccines in large quantities at low prices. What is the current progress of vaccine development? How does the C1 differentiate it from other platforms or technology, e.g., Moderna.
We have several ongoing COVID-19 vaccine development programs with different partners, including the Frederick National Laboratory in the US, which is related to NIH, as well as the IIBR, Israel Institute For Biological Research, to express a number of their vaccine candidates. We are also working with a group of vaccine experts and scientists in Europe from University Utrecht, TiHo University, and Erasmus Medical Center, in developing our own receptor-binding domain (RBD) based SARS-CoV-2 vaccine candidate. The C1 expressed SARS-CoV-2 RBD vaccine is produced at extremely high yields and is more similar to mammalian glycan structures than insect cells (i.e., baculovirus) or yeast. The IIBR has already shown in their mice trials that the C1 expressed SARS-CoV-2 RBD has excellent immunogenic protection and creates high levels of neutralizing antibodies. In conjunction with the EU scientists, we expect to conduct hamster trials on the C1 expressed SARS-CoV-2 RBD very soon. Including the recently concluded IIBR mice study and the upcoming EU hamster trial, we anticipate that more than six different animal trials using the C1 SARS-CoV-2 expressed RBD with and without nanoparticles, including mice, hamsters and transgenic ACE2 mice, will be completed by the end of 2020 by five different organizations.
Moderna’s and other vaccines are based on mRNA, which is a new technology more similar to gene therapy. While they appear to be making good progress, keep in mind that there are currently no US approved DNA or RNA vaccines for any antigen.
Our C1 expressed vaccine candidates are based on the expression of recombinant antigens. Production of recombinant protein vaccines have been shown to be safe and effective. Many experts believe this method stands a higher likelihood of success in preventing the COVID-19 virus. C1 is a very productive production host, and among several advantages is its ability to produce very large quantities of antigens rapidly, more affordably at flexible commercial scales using existing microbial fermenters. We are offering access to our C1 gene expression platform to others, in addition to using it in our own vaccine programs with our current collaborators such as the IIBR, European scientists, and the US Frederick National Lab. We believe our C1 cell line can help to more rapidly and more affordably eradicate the COVID-19 pandemic, be better prepared for future pandemics or if the virus mutates which may require new vaccine and antibody candidates to be developed and manufactured. C1 can also help to bring the cost of the very expensive biologic drugs down to make healthcare more accessible and affordable to patients globally.
Dyadic’s goal is to enable volume production globally at an affordable price with lower CapEx requirements. The world population is about 7.8 billion and in order to get the pandemic under control, it’s going to require massive production of vaccines and antibodies on a scale never before seen and at a low enough cost to make it affordable on a global basis. It is widely thought that the manufacturing process could be a bottleneck for COVID-19 vaccines and antibodies, and we believe our C1 gene expression platform provides a commercially viable solution to this problem.
MyBioGate: After collaborating with Hengrui, how will Dyadic use C1 to help Hengrui develop biological drugs?
We are very excited about this collaboration with Hengrui. Hengrui shares Dyadic’s vision of bringing low-cost biologic medicines to benefit patients, as their R&D President, Dr. Lianshan Zhang commented. We expect this collaboration with Hengrui will help introduce C1 as a game-changing protein production host and validate the capability of C1 for gene discovery, development, expression, and manufacture. Our goal is to further improve our C1 platform along the way, and work with Hengrui to apply C1 to many more Hengrui biologic drug candidates in the future.
MyBioGate: Dyadic had successfully licensed the C1 expression system to renowned companies such as DuPont, Abengoa, BASF, and Codexis. Any differences or challenges when discussing a partnership with Chinese companies?
China is a huge market and we are impressed by the speed and growth of its healthcare and biopharmaceutical industry. We want to introduce our proprietary C1 protein expression platform to more Chinese pharmaceutical companies and healthcare organizations and enter into additional partnerships, collaborations, and license agreements with them.
The differences in China relate to intellectual property protection as well as cultural and language barriers. However, we have extensive, long-term relationships in Europe and other areas outside the US which gives us a certain experience to bridge these gaps. We see China as an exciting market and a great opportunity for Dyadic. We see the Chinese culture being closely aligned with Dyadic’s entrepreneurial spirit, like Nike’s slogan says, together let’s just “Do It” to fulfill our obligation to make healthcare an inalienable right to all Chinese citizens as well as all the citizens globally.
MyBioGate: Dyadic and Hengrui met for the first time at the China Focus Conference. How will this collaboration help you enter the China market? How do you view the market potential of China in healthcare?
Jiangsu Hengrui is a well known pharmaceutical company in China. The collaboration with Hengrui definitely helps to establish further the credibility of C1 and Dyadic in China and elsewhere. It’s an excellent start for us to enter the China market. We also have a non-exclusive research license with WuXi Biologics, the leading CDMO in China, and even globally, to evaluate our C1 in their cGMP facility.
We expect these programs coupled with the many other programs we have ongoing with top-tier Western and Asian pharmaceutical and biotech companies will help us to introduce C1 to more Chinese companies. We look forward to working with more Chinese pharma and biotech companies in the future by applying our technology to help reduce costs throughout the healthcare system and make biologic drugs more accessible and affordable to patients. We believe there is tremendous potential in the healthcare market in China, and we expect to be an active participant in collaboration with our partners.

0 Comments