Author: HE Sheng
Lung cancer is one of the most common types of malignant tumors in the world. It has become the primary cause of death from malignant tumors in China’s urban population. According to statistics from the WHO official website, in 2018, there were nearly 2.1 million newly diagnosed lung cancer cases worldwide and 1.76 million deaths.
In recent years, the incidence of non-small cell lung cancer has been increasing, and it has become the tumor with the highest fatality rate both in China and the world.
The treatment of advanced non-small cell lung cancer is first divided into targeted therapy and non-targeted therapy according to the presence or absence of driver gene mutations. In the last article, I introduced the primary therapies used to treat NSCLC. In this article, I will focus on RET inhibitors.
In the latest 2020 V5 non-small cell lung cancer treatment guidelines, based on the detection of the four conventional driver genes of advanced non-small cell lung cancer—EGFR, ALK, ROS1, and BRAF—the detection of MET gene 14 mutation and RET gene fusion were added. The detection of RET resulted in the necessary checking of six genes. Selpercatinib, which was approved by the FDA in May of this year, is recommended as the priority treatment for patients with RET gene fusion non-small cell lung cancer.
The RET gene and NSCLC
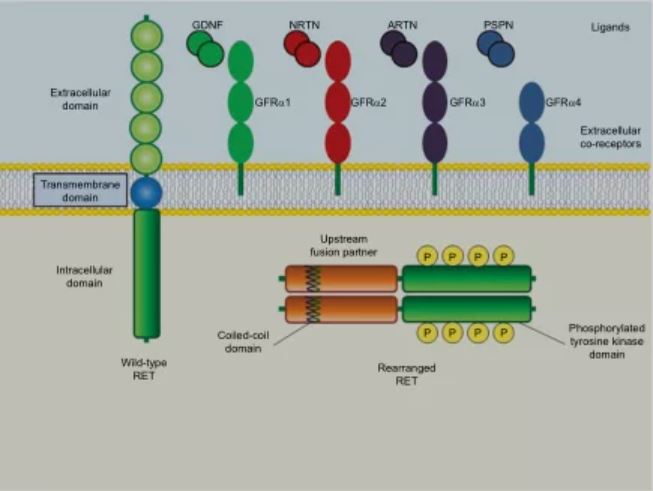
▲Figure 1: Schematic structure of wild-type and rearranged RET proteins in cancer cells
Source: Giuseppe, Bronte, Paola, et al. Targeting RET-rearranged non-small-cell lung cancer: future prospects
RET fusion and mutation are key disease drivers of many cancer types. Scientists discovered first that the RET gene is related to the occurrence of papillary thyroid cancer. In 2012, researchers also discovered this mutation in patients with non-small cell lung cancer. RET gene fusion or mutation is one of the newly discovered non-small cell lung cancer driving oncogenes.
Data show that approximately 1% to 2% of NSCLC patients experience RET fusion, while approximately 10% to 20% of patients with papillary thyroid cancer and approximately 90% of patients with advanced medullary thyroid cancer experience RET mutations. In addition, in patients with colorectal cancer, breast cancer, pancreatic cancer and others, there is also small probability of RET gene changes. RET can also be observed at low frequency in EGFR mutant NSCLC patients who are resistant to targeted drug therapy.
In the past, RET-positive non-small cell lung cancer patients did not have approved targeted drugs, but this year, two RET inhibitors were launched successively, which finally brought a revolution in the treatment of these patients.
Pralsetinib (BLU-667) and Selpercatinib (LOXO-292), both have strong clinical safety results. Compared with conventional treatment, the tumor remission rate is significantly improved. What is commendable is that even patients who have undergone multiple treatments and patients with brain metastases have obvious effects. The NCCN guidelines have recommended Selpercatinib as the priority drug for patients with RET non-small cell lung cancer.
RET small molecules bring new treatment options
Selpercatinib: the first RET inhibitor to treat NSCLC
In May 2020, the FDA approved the RET inhibitor Retevmo (Selpercatinib, 40 mg & 80 mg capsules) developed by Eli Lilly for the treatment of adult patients with RET-positive metastatic NSCLC and RET-positive advanced thyroid cancer. This drug is the first RET inhibitor approved by the FDA for the treatment of non-small cell lung cancer and has been specified as a standard therapy by NCCN guidelines.
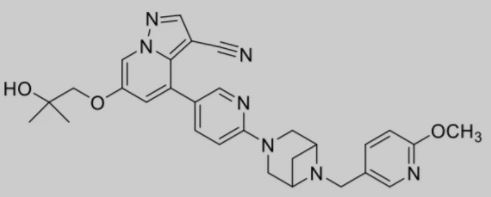
▲Figure 2: Selpercatinib chemical structure
Selpercatinib’s approval was based on the positive results from the clinical I/II trial LIBRETTO-001. 105 RET fusion-positive adult NSCLC patients who received platinum-based chemotherapy had an ORR of 64% and a median duration of response (DOR) of 17.5 months. The proportion of patients with a DOR of more than 6 months was 81%. However, for 39 RET fusion-positive NSCLC patients who had never received treatment, the ORR was 84%, and the proportion of patients with a DOR of more than 6 months was 58%.
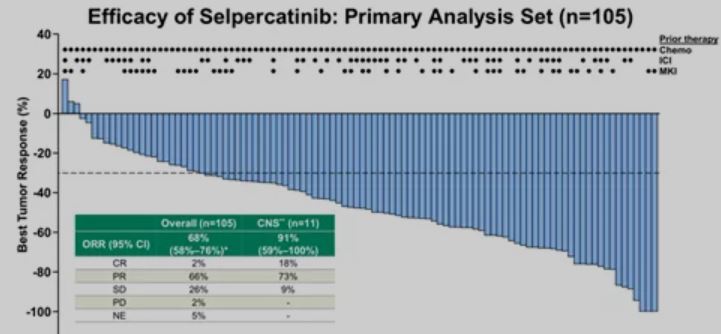
▲Figure 3: Efficacy of selpercatinib on RET fusion non-small cell lung cancer
Source: Drilon A, Oxnard G, Wirth L, et al. PL02.08 Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers
In addition, the drug is very well tolerated, and the drug-related toxicity caused a withdrawal rate of only 2%. The most common side effects were hypertension and elevated liver function.
Retevmo entered clinical trials in May 2017 and was approved in less than three years, representing the fastest time for the development of oncology drugs with multiple indications.
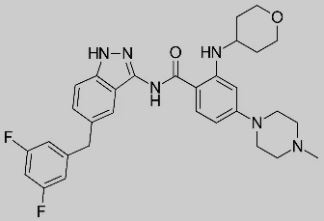
Pralsetinib, a highly effective RET inhibitor
On September 4, 2020, Blueprint Medicines, a company dedicated to the development of precision therapy for rare diseases and cancer immunotherapy, announced that the US FDA has accelerated the approval of the company’s small molecule RET inhibitor Gavreto (pralsetinib) for the treatment of RET fusion-positive adult patients with small cell lung cancer (NSCLC). According to the cooperation agreement reached between Blueprint Medicines and Roche, Blueprint Medicines and Roche’s Genentech will jointly market Gavreto in the United States.
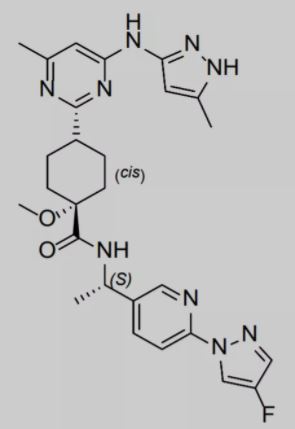
▲Figure 4: Pralsetinib chemical structure
For patients in China, such drugs are still only available on the market abroad. The difficulty of seeking medical attention, the difficulty of obtaining medicine, the price of medicine and other factors will become obstacles for patients.
Fortunately, in China, CStone Pharmaceuticals has obtained the development and promotion rights of this innovative therapy. This product is currently in clinical phase II/III phase for the treatment of non-small cell lung cancer in China.
Gavreto is a once-daily oral RET targeted therapy developed by Blueprint Medicines. It can specifically and powerfully inhibit the RET mutation that drives many cancer types. Compared with Vandetanib, Cabozantinib and RXDX-105, the lethality of Gavreto to wild-type RET is increased by 8 to 28 times. Data from preclinical studies have shown that compared to other related kinases such as VEGFR2, FGFR2 and JAK2, Gavreto can inhibit RET mutations at a lower concentration. In addition, the fusion type of RET does not affect the curative effect, whether or not to receive PD-1/PD-L1 before does not affect the curative effect, and whether or not brain metastases does not affect the curative effect.
▲Figure 5: Gavreto
Source: Blueprint Medicines website
This approval is based on data from the clinical I/II clinical trial ARROW, which shows that Gavreto has curative effect on both newly treated and treated RET fusion-positive NSCLC patients, without considering the RET fusion site or central nervous system involvement. Key clinical trial data showed that among 87 patients who had previously received platinum-containing chemotherapy, the overall response rate (ORR) of patients treated with Gavreto was 57%, and the complete response rate (CR) was 5.7%. Among 27 newly treated patients who were not suitable for platinum-containing chemotherapy, the ORR was 70% and the CR was 11%.
In terms of safety, the most common adverse reactions to Gavreto are fatigue, constipation, musculoskeletal pain and increased blood pressure.
Targeted therapies have significantly improved the treatment of patients with non-small cell lung cancer driven by oncogenes (including EGFR and ALK). The approval of the specific RET inhibitor Praalsetinib marks another milestone in precision medicine. This approval may change the standard of treatment for patients with positive RET fusion non-small cell lung cancer.
Multi-target drug RXDX-105
RXDX-105 is a powerful RET inhibitor developed by Ignyta, which was later acquired by Roche. Although it retains the VEGFR target, its inhibitory ability to VEGFR is far weaker than RET.
A phase I/II clinical trial evaluating it for the treatment of non-small cell lung cancer included 40 patients. The results showed that the ORR was 19%. Patients with KIF5BRET fusion did not have any response, while ORR in patients without KIF5B-RET fusion was 69%, suggesting that selecting patients according to the type of rearrangement has a relevant effect. The most common G3 adverse events did not exceed 10%, and no G4 toxicity was reported.
▲Figure 6: RXDX-105 chemical structure
References
【1】Giuseppe, Bronte, Paola, et al. Targeting RET-rearranged non-small-cell lung cancer: future prospects. Lung Cancer, 2019.
【2】Drilon A, Oxnard G, Wirth L, et al. PL02.08 Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers. Journal of Thoracic Oncology, 2019, 14(10): S6-S7.
【3】WHO official website
【4】Eli Lilly’s official website
【5】Blueprint Medicines official website
【6】NCCN official website: NCCN clinical practice guidelines in oncology: Non-small cell lung cancer version 5.2020

0 Comments