Author: HE Sheng
Recently the CDK4/6 inhibitor Cosela (trilaciclib), developed by G1Therapeutics, was launched in the United States. The inhibitor’s aim is to “decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for extensive-stage small cell lung cancer (ES-SCLC)”. The product is expected to enter the market in March.
Trilaciclib is a short-acting CDK4/6 inhibitor that is to be used before chemotherapy. It causes the cell cycle of bone marrow stem cells to stop so that they will not be harmed by chemotherapy drugs. Trilaciclib has been granted breakthrough therapy designation by the FDA.
▲Trilaciclib structure
On August 3, 2020, Simcere Pharmaceuticals announced that it signed an exclusive licensing agreement to G1’s trilaciclib development and commercialization rights for all indications in Greater China. It also will participate in global clinical trials for the inhibitor.

According to the terms of the agreement, G1 will receive an initial payment of US$14 million with up to US$156 million in development and commercialization milestone payments. Meanwhile, Simcere Pharmaceuticals will pay G1 royalties based on trilaciclib’s annual net sales in Greater China.
In recent years, with the frequent changes in the pharmaceutical industry, the market has become more open. A trend has emerged of frequent alliances between Chinese and foreign pharmaceutical companies to jointly develop Chinese and international pharmaceutical markets.
Trilaciclib is the first product approved by the FDA to protect bone marrow cells from damage caused by chemotherapy. This variety is undergoing clinical research on various indications in the United States, including for breast cancer and bladder cancer. Currently, R&D in China is in preparation for the IND application.
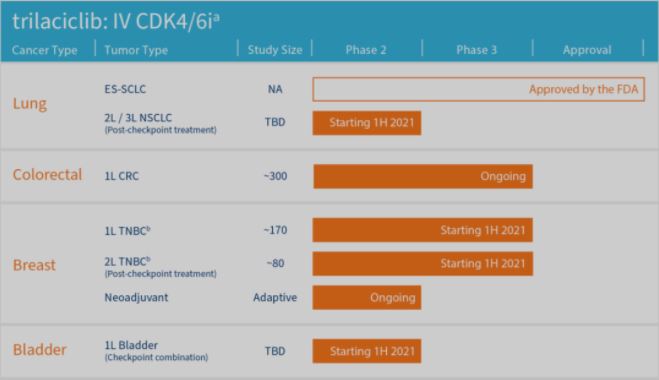
▲Trilaciclib stage IV clinical trial results
This approval is based on three randomized, double-blind, placebo-controlled pivotal studies in patients with extensive-stage small cell lung cancer (Study 1: G1T28-05; Study 2: G1T28-02; Study 3: G1T28-03) . These studies randomly assigned 245 patients to receive intravenous Cosela injections or a placebo before chemotherapy. In all three studies, patients who received Cosela had a lower chance of developing severe neutropenia than patients who received the placebo. Among those patients with severe neutropenia, on average, those who received Cosela had a shorter duration of symptoms.
The main adverse reactions are fatigue, hypocalcemia, hypokalemia, and hypophosphatemia.
Small cell lung cancer is an aggressive disease, which tends to spread faster than non-small cell cancer. Usually there are no initial symptoms. Once symptoms appear, it often indicates that the cancer has spread to other parts of the body. Approximately 70% of patients are diagnosed when the cancer has metastasized. The 5-year survival rate for small cell lung cancer is 12%-15%, with longer term survival rate at less than 2%. Chemotherapy is a common treatment for small cell lung cancer.
One of the most common side effects of chemotherapy is myelosuppression, or bone marrow suppression. Because bone marrow stem cells are killed by chemotherapy drugs, it can lead to serious consequences such as anemia, neutropenia, or thrombocytopenia. These complications will affect the patient’s quality of life and prognosis.
So far, methods to inhibit bone marrow suppression include the use of growth factor preparations, the use of antibiotics, and blood transfusions. In contrast, trilaciclib has a unique mechanism of action and is the first new therapy to protect bone marrow from chemotherapy damage.
This is a new method to protect hematopoietic stem cells and progenitor cells in the bone marrow from chemotherapy damage. Such a method can help reduce some chemotherapy-related toxicity, make chemotherapy safer and more tolerable, and also reduce the need for reactive rescue intervention.
Sources
- FDA website
- Simcere website
- G1 Therapeutics website

0 Comments