Author: HE Sheng
Parkinson’s disease is the second most common neurodegenerative disease after Alzheimer’s disease. It is caused by low levels of dopamine in the patient’s brain. As the disease progresses, the production of dopamine steadily decreases, leading to a gradual increase in motor symptoms, including slow movement, tremors, and impaired balance.
According to estimates by the Parkinson’s Disease Foundation, there are more than 10 million patients worldwide who suffer from the disease, including more than 1 million in the United States. In China, the prevalence of Parkinson’s disease has increased from 76 per 100,000 to 180 per 100,000 in recent decades. The likelihood of getting Parkinson’s disease increases with age with its prevalence almost doubling in the elderly compared to other age groups. According to the 2017 data of Global Health Data, there are about 2.5 million Parkinson’s disease patients China. As the population ageing accelerates, it is estimated that by 2030, there will be about 5 million Parkinson’s disease patients.
There is currently no cure for Parkinson’s. The management of this disease mainly uses dopamine to control motor symptoms. The current gold standard therapy for motor symptoms is levodopa and carbidopa.
The drug treatment of Parkinson’s disease can be divided into six categories: anticholinergics, amantadine, dopamine replacement therapy, dopamine receptor agonists, monoamine oxidase-B inhibitors, and COMT inhibitors. They realize the treatment of Parkinson’s disease through different pharmacological mechanisms.
According to the “Chinese Parkinson’s Disease Treatment Guidelines: Third Edition” patients younger than 65 years old and without mental impairment can choose between the following treatments:
- Non-ergot dopamine receptor (DR) agonists
- Monoamine oxidase B (MAO-B) inhibitor or vitamin R
- If tremors are obvious and other anti-Parkinson drugs are not effective, patients may choose anticholinergic drugs such as trihexyphenidyl
- Compound levodopa is generally used when earlier treatments have not been effective
- Compound levodopa + catechol-O-methyltransferase (COMT) inhibitor
For patients over 65 years of age, compound levodopa is the first choice, and dopamine receptor agonists, MAO-B inhibitors, COMT inhibitors can be added when necessary. Anticholinergic drugs are generally avoided as they have more side effects.
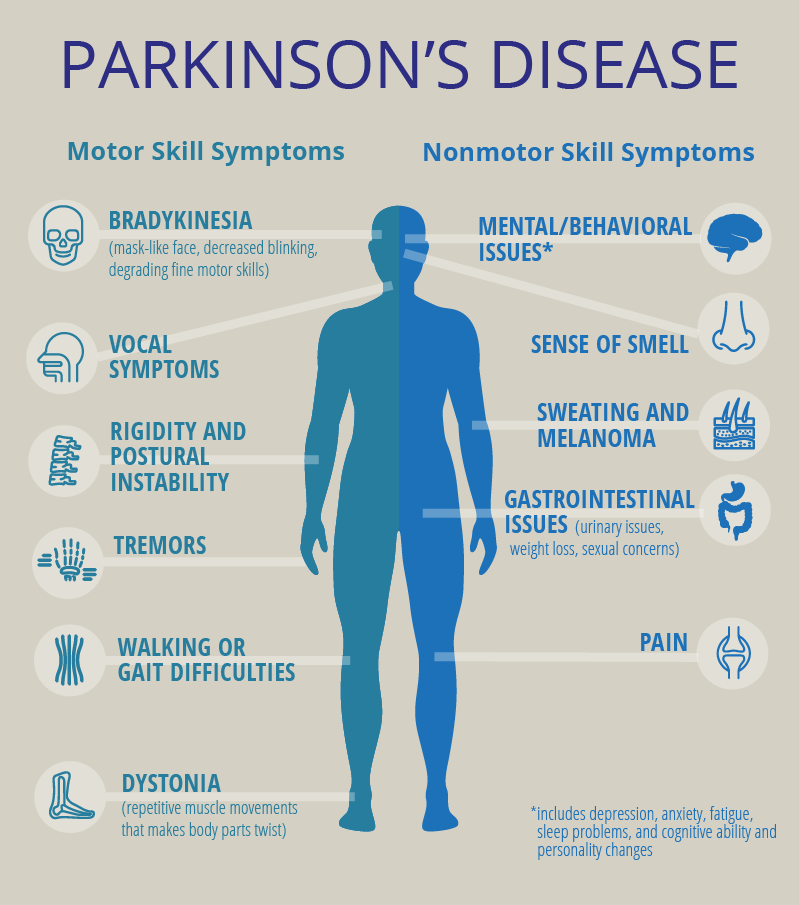
▲ Figure 1: Symptoms of Parkinson’s Disease
Source: pcori (Patient-Centered Outcomes Research Institute)
Market size for Parkinson’s disease treatments
According to IMS data, the global PD drug market has been almost flat in recent years, maintaining between US$3.5-4 billion. With the passage of time, the market share of levodopa compound preparations has gradually increased, and the share of MAO-B inhibitors has gradually decreased. By 2019, the global PD market was close to US$3.7 billion.
DR agonists and levodopa compound preparations are currently part of the mainstream PD drugs, accounting for about 80%. KDT-3594 is a new type of oral non-ergot dopamine agonist. DR agonists are currently the best anti-PD drug in the global market.
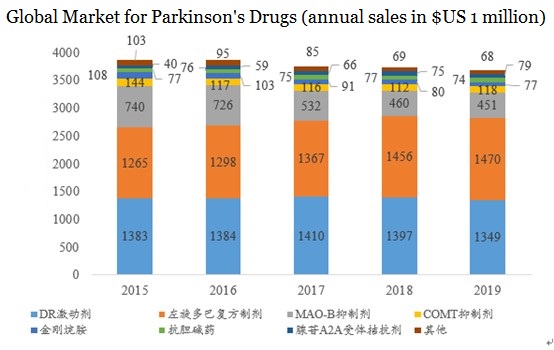
▲ Figure 2. Distribution of global Parkinson’s drugs in 2019
Source: Yaodu
Note: Light blue is dopamine receptor (DR) agonists, blue is amantadine, orange is levodopa, green is anticholinergics, grey is MAO-B inhibitors, dark blue is adenosine A2A agonist, yellow is COMT inhibitors, red is other
Europe, the United States, and Japan are the main markets for Parkinson’s disease treatments. The Chinese PD market only accounts for 5% of the global market. However, Chinese PD patients account for 50% of the global market. This shows that China has huge market potential and more innovative varieties are urgently needed there.
Among the top 10 varieties of public hospitals and zero-sale terminals in China in 2019, pramipexole ranked first in sales. This variety was originally developed by Boehringer Ingelheim and was listed in China in August 2014. As the first domestic pramipexole hydrochloride tablet that passed consistency evaluations, CSPC’s Enxi® will officially enter the field of Parkinson’s disease at a lower price. The launch of Enxi® will bring more treatment options to clinics and greatly ease the socio-economic pressure of R&D due to price issues.
The second highest selling product in China is dopaserazine. The drug is a 4:1 compound preparation of levodopa and benserazide and is considered the gold standard for the treatment of Parkinson’s disease. The original research manufacturer is Roche but there are many generic drugs on the market in China. These include products by Fuda Pharmaceutical, YSY (Shanghai) Pharmaceutical, Guangzhou Baiyunshan Pharmaceutical Holdings, and Aida Pharmaceutical.
In addition, varieties such as entacapone, pirbedil and trihexyphenid also showed a good market share.
CBC Group invests in company developing a treatment for Parkinson’s
Founded in 2014, CBC Group is a dual-currency investment fund dedicated to the R&D and commercialization of innovative drugs and with an investment focus on medical companies and service industries. Its direct asset management exceeds US$2 billion, while capital that can be leveraged through incubation holdings exceeds US$10 billion. According to statistics from PrivateEquity International (PEI), an international private equity industry magazine, CBC Group is the largest healthcare professional investment fund in Asia, ranking sixth in the world. This private equity fund focuses on platform construction and acquisition opportunities in the three core areas of biomedicine, medical technology and medical services. Its investment fields include biopharmaceuticals, drug distribution, molecular diagnostics, medical devices and medical services.
Fu Wei, the founder of CBC, said that for the companies incubated by CBC, any transaction can make give CBC 5-10 times return on their investment. Meanwhile, the domestic Chinese Parkinson’s (PD) market has exceeded RMB 2 billion. Affamed Therapeutics Limited, a company incubated by CBC, recently introduced a new Parkinson’s treatment called KDT-3594. If this product is successfully listed, the profits for CBC could be high.
Affamed introduces a new treatment for Parkinson’s disease
On October 9, 2020, Affamed Therapeutics (headquartered in Hong Kong), a pharmaceutical company incubated by CBC, was authorized to obtain the Parkinson (PD) new drug KDT-3594 from the Japanese pharmaceutical company Kissei Pharmaceutical in mainland China, Taiwan, Hong Kong, Macau with exclusive rights for development and commercialization in six southeast Asian countries: Singapore, Malaysia, Thailand, Indonesia, Vietnam and the Philippines. According to the agreement, Kissei will collect an advance payment from Affamed, milestone payments based on the development stage, and royalties based on product sales.
KDT-3594 is a new type of oral non-ergot dopamine agonist. Its role is to stimulate the dopamine receptors in the basal ganglia, thereby reducing the symptoms of Parkinson’s disease caused by insufficient dopamine. KDT-3594, as a new therapeutic agent for Parkinson’s disease, could also reduce the risk of typical side effects of existing ergot and non-ergot dopamine agonists. KDT-3594 is currently undergoing phase II clinical trials in Japan.
Summary
There are 10 million Parkinson’s patients worldwide, and the overall prevalence of people over 65 in China is 1700 per 100,000. The incidence of the disease increases with the aging of the population, which brings a heavy burden to the family and society. It is expected that global pharmaceutical institutions, especially domestic institutions, will increase investment in this field and bring more treatment options for PD patients.
References
- CBC Group’s official website
- KisseiPharmaceutical official website
- NIH official website
- Yaodu data
- IMS data

0 Comments